Elements Would You Expect to Lose Electrons in Chemical Changes
An atom has 3 protons 4 neutrons and 3 electrons. Check all that apply.
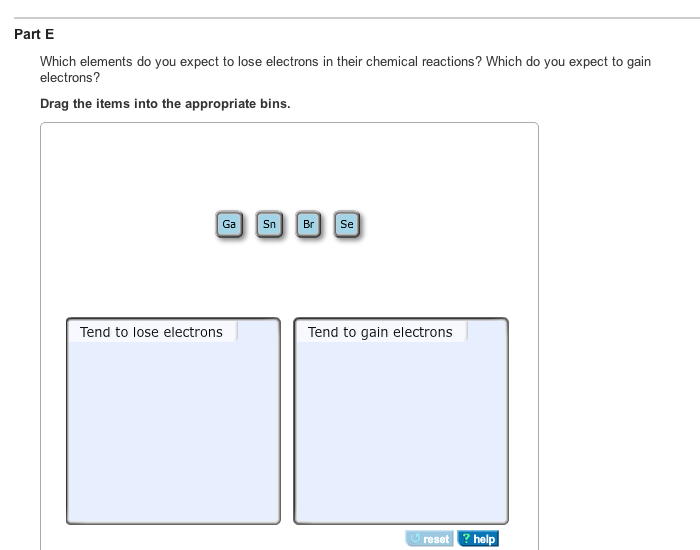
Solved Which Elements Do You Expect To Lose Electrons In Chegg Com
A copper ion with a 2 charge.

. I would expect the nonmetals fluorine and sulfur to gain or share electrons depending of the. Sorry during a chemical change such as in the formation of an ionic compound are metals. The cation produced in this way Na is called the sodium ion to distinguish it from the element.
Classify the following elements based on whether or not would you expect them to gain electrons in chemical changes. 100 1 rating Metals are electropositive in nature - they loose electrons. View the full answer.
Loss of 7 electrons. We review their content and use your feedback to keep the quality high. They typically form cat ions so anything that is a metal you would expect it to lose.
Which of the following elements would you expect to lose electrons in chemical changes. The elements that typically lose electrons during a physical change. A selenium atom Se would form its most stable ion by the 1.
Sort these Items Into the proper categories Would expect to lose electrons Wouldnt expect to lose electrons. Which of the following elements would you expect to lose electrons in chemical changes. A neutral sodium atom is likely to achieve an octet in its outermost shell by losing its one valence electron.
Wouldnt expect to lose electrons. I would expect the metals potassium barium and copper to lose electrons during chemical reactions. Would expect to gain electrons.
The elements that you would expect to gain electrons in a chemical change. Which elements would you expect to lose electrons in chemical changes. Check all that apply-copper-barium-fluorine-sulfur-potassium.
Which elements would you expect to lose electrons in chemical changes. Gain of 2 electrons. Sort these Items Into the proper categories Would expect to lose electrons Wouldnt expect to lose electrons.
471 Na Na e. Which of the following elements would you expect to lose electrons m chemical changes. Would expect to lose electrons.
Gain of 1 electron. What is the total number of electrons present in each ion. Which of the following elements would you expect.
Our non metals non metals typically gain electrons and become an ions during the formation of an ionic compound so the non metals in the list that is provided include. Which of the following elements would you expect to lose electrons in chemical changes. The outermost shell of the sodium ion is the second electron shell which has eight electrons in it.
Up to 256 cash back Get the detailed answer. Which of the following elements would you expect to lose electrons in chemical changes. Help please and tell me how you got the answer.
A molybdenum ion with a 4 charge. Loss of 2 electrons. Loss of 1 electron.
Experts are tested by Chegg as specialists in their subject area. Predict how many electrons are in each ion. Electrons potassium barium and copper are the three medals.
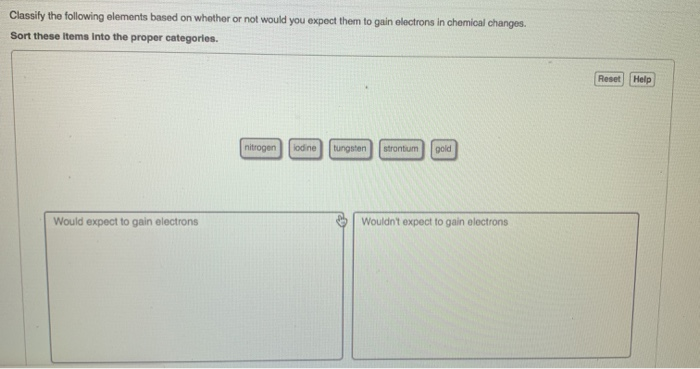
Solved Classily The Following Elements Based On Whether Or Chegg Com

Solved 44 Which Elements Would You Expect To Lose Electrons Chegg Com
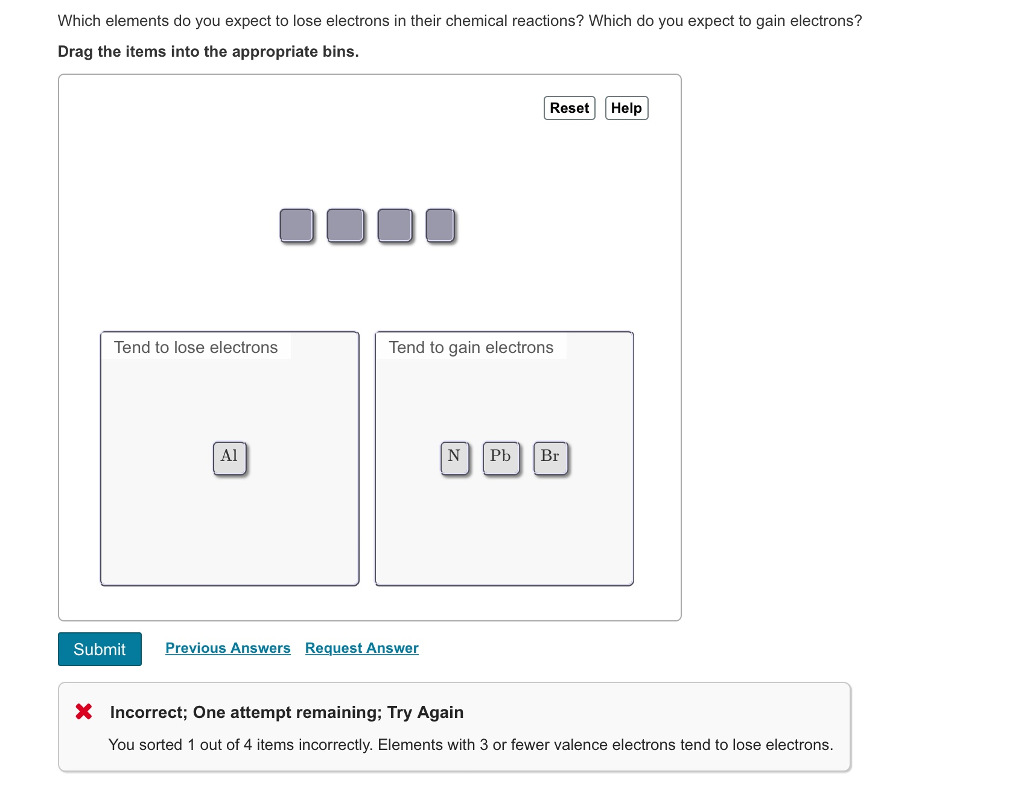
Solved Which Elements Do You Expect To Lose Electrons In Chegg Com
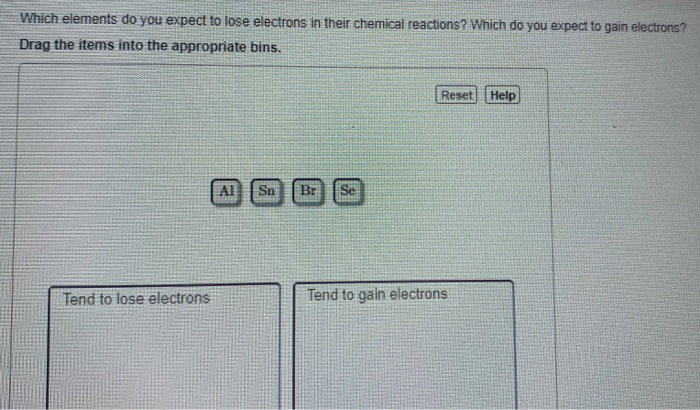
Solved Which Elements Do You Expect To Lose Electrons In Chegg Com
Comments
Post a Comment